What does A mean in BRITISH MEDICINE
Mass number is an abbreviation found in the fields of chemistry and physics. It is important to understand what this term means and how it is used. This article will explain the meaning of the designation "A" for mass number and provide relevant FAQs about the concept.
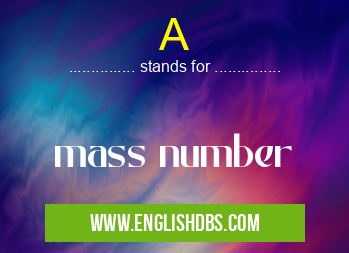
A meaning in British Medicine in Medical
A mostly used in an acronym British Medicine in Category Medical that means mass number
Shorthand: A,
Full Form: mass number
For more information of "mass number", see the section below.
Essential Questions and Answers on mass number in "MEDICAL»BRITMEDICAL"
What does "A" stand for in mass number?
The letter "A" stands for the term 'atomic mass' or 'atomic weight'. It represents a particular atom's total number of protons and neutrons, or its nucleon count.
How is the mass number determined?
The mass number of a particular atom is calculated by adding up the total number of protons and neutrons within its nucleus. It can also be found on periodic tables of elements which list atomic masses and symbols.
Is A always equal to the sum of protons plus neutrons?
In most cases, yes. However, there are certain isotopes where A may not be equal to the sum due to differences in binding energies between protons and/or neutrons.
Does mass number determine an element's identity?
No - mass numbers only tell us how much matter each type of atom contains; they do not identify what element it is nor determine its physical properties or chemical reactivity.
Can more than one element have the same mass number?
Yes - different isotopes of an element can have the same atomic mass due to having different numbers of neutrons but still having the same amount of protons as other isotopes.
Final Words:
In summary, molecular weight (mass number) notation uses “A†as an abbreviation for atomic weight or atomic mass, which refers to a particular atom's nucleon count (the sum of all its protons plus neutrons). Mass numbers help us determine not only how much matter each type of atom contains, but also which isotopes exist in nature.
A also stands for: |
|
| All stands for A |
